| <Home page> | <Invention> | <Bio> | <Links> |
Abstract: In this paper we describe the design of a strategy based on a number of selection rules that could potentially lead us to the identification of materials that will replace mercury and play the role of the active medium in low pressure discharge lamps. The selection rules focus on the emission and thermodynamic characteristics of species as well as safety considerations and the strategy is by no means a complete solution but a logical approach to the solution based on available sources and databases.
Invention |
1. Introduction
Humanity has to deal with two main issues regarding energy. The first is the availability of non-sustainable energy sources and whether the global demand for energy can be met. This is due to their depletion in certain parts of the world or due to geopolitical factors and in any case the impact to the global economy is substantial. The second issue is one that deals with the environmental changes of the planet and the impact of these changes to our lives. The burning of fossil fuels as the most common energy generation mechanism results in the formation and emission of carbon dioxide as a byproduct which is one of the gases responsible for the greenhouse effect.
Considering than man is using about a fifth of the world’s generated electric energy for lighting applications it is easy to appreciate the importance of light source technology both on an economic and an environmental level. Light sources and lighting not only represent an economic market of billions of dollars but the consumption of energy for lighting is responsible for the generation of billions of tons of CO2 gas annually.
Light source technology to this day has been dominated by the technology of incandescence, electrical discharges and light emitting diodes. One may acknowledge the attractive features of incandescent lamps such as the high color rendering index of the white light they emit and therefore the near perfect color reproduction of the illuminated objects or the robustness and long life of the light emitting diodes but the fact is that they lag far behind when it comes to efficiency compared to the technology of the electrical discharges. Incandescent lamps and products based on LEDs still produce less than 20 lumens per watt while products based on electrical discharges produce more than 40 lumens per watt, reaching a value of 200 in the case of low pressure sodium discharges.
Without a doubt mercury plays a central role in the market of light sources as an element to be found in the majority of discharge lamps from low pressure mercury fluorescent lamps to high pressure high intensity discharges (HID) to super high pressure projection lamps. Even in high pressure sodium lamps and high pressure metal halide lamps, mercury is usually added and acts as one of the main active media.
The light source scientists and engineers today are asked to not only push the upper limit of light source efficiency to higher values but also to eradicate mercury and search for new material that will serve as efficient radiators. The replacement of mercury in lighting products is a requirement that stems from a long lasting environmental concern towards mercury, particularly its organic form that is hazardous and life threatening, and has found a new frame these days in the form of government official directives (the well known RoSH directives where the acronym stands for Reduction of Hazardous Substances). Apart from mercury, the use of lead, cadmium and hexavalent chromium is also not allowed.
For that reason the authors have taken a fresh look at the existing light source technologies and have decided to focus on the evolution if not reinvention of electrical discharges as light sources as it this technology that needs desperately new materials to act as the active media and replace old ones. The target for the efficiency and/or efficacy is of course for all scientists in the field, to reach the same value or higher than the one achieved via the use of mercury in the discharge tubes (in low pressure discharge tubes containing mercury this is roughly 65% efficiency and the output can reach 120 lm/W for certain lamps but there is usually a trade off between efficacy and color rendering index depending on the chosen phosphor for different applications).
In this review paper the authors have devised a set of selection rules according to their own personal views and through the scanning of various databases and elimination steps, they have come up with a list of candidate materials which they believe have the potential to become the active media of new lighting products.2. The selection criteria
2.1. The candidate species
The matter of fact is that the use of pure elements as active media poses the limitation of insufficient vapor pressure in most cases with few exceptions that have already been tested and are well known. Elements like the rare gases, mercury and sodium have already been the base for a number of products while other elements with useful emission properties can only be used in molecular form and in high pressure lamps where the temperatures reach high enough values for them to be vaporized and dissociated to a useful degree.
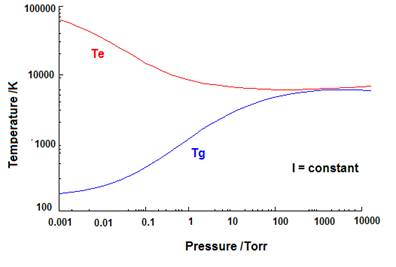
Gas and electron temperatures of discharges with respect to the fill gas pressure.
In our search we will focus on species whose emission properties satisfy our predefined range and which will include not only atoms but also diatomic species (so as to overcome at least partly, the vapor pressure limitations of the elements), with at least one of the atoms being an element with useful emissions due to the fact that even with molecules in the gas phase, significant atomization occurs and the atomic emissions contribute significantly to the overall radiated power output. Finally one should search for parent molecules if the diatomic species are not freely available or stable in their form.
2.2. The spectral range
Κάτω από τις συνθήκες λειτουργίας ενός λαμπτήρα εκκένωσης χαμηλής πίεσης ατμών υδραργύρου (εναλλασσόμενο ρεύμα συχνότητας 50 Hz όταν ηλεκτρομαγνητικά στραγγαλιστικά πηνία χρησιμοποιούνται έως μερικές δεκάδες kHz με ηλεκτρονικά όργανα λειτουργίας) η γραμμή εκπομπής υδραργύρου με τη μεγαλύτερη ένταση είναι αυτή με μήκος κύματος τα 254 nm. Η γραμμή αυτή εκπομπής είναι γνωστή και ως γραμμή συντονισμού αφού η κβαντική (ηλεκτρονική) μετάβαση που τη δημιουργεί είναι από το πρώτο ενεργειακό επίπεδο προς το θεμελιώδες επίπεδο του ατόμου. Για τη δημιουργία χρήσιμου φωτός για φωτισμό, μια φωσφορίζουσα πούδρα εφαρμόζεται στα εσωτερικά τοιχώματα του σωλήνα η οποία θα απορροφήσει τα φωτόνια με την υπεριώδη ενέργεια και κατόπιν απενεργοποίησης της πούδρας θα απελευθερώσει φωτόνια με ενέργεια στο ορατό μέρος του φάσματος. Μέσω αυτής της διαδικασίας βέβαια χάνεται ενέργεια αφού ανταλλάσετε ένα υπεριώδες φωτόνιο για ένα ορατό. Η απώλεια αυτή ενέργειας ονομάζεται απώλεια Stokes και δεν περιορίζεται μόνο στην περίπτωση του υδραργύρου αλλά λαμβάνει χώρα με κάθε ενεργό μέσο όταν αυτό εκπέμπει σε ενέργειες εκτός του ορατού φάσματος και πρέπει να γίνεται μετατροπή μήκους κύματος με τη χρήση ενός φωσφορίζων υλικού.
Under the normal conditions of operation (from mains frequency AC when electromagnetic ballasts are used to a few tens of kilohertz with electronic gear) the most intense emission line of mercury is at 254 nm which is also known as the resonant line and it is the result of an electron transition from the first atomic excited state to the ground state. In order to produce useful light for lighting a phosphor must be applied on the inner walls of the discharge tube that will absorb the UV photons and after relaxation will emit visible photons. This process introduces an energy loss mechanism known as Stokes losses which is not only associated with mercury but with any active medium whose emission photons have to be converted to visible light with the use of a phosphor powder. The efficiency of a lamp is defined as the radiated power over the total electrical power supplied to the lamp but the efficacy of a lamp is defined as the power emitted in the visible range over the total power input. When measuring the efficacy though, one has to take into account the human eye’s sensitivity to the different wavelengths of the visible range which peaks around 555 nm.
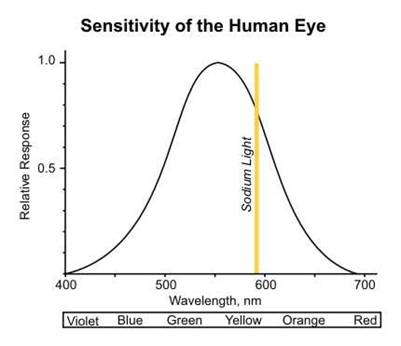
Sodium’s resonant line lies around the peak of the human eye’s sensitivity curve (589 nm).
One of the reasons the low pressure sodium lamp has a high efficacy is due to the fact sodium’s resonant line lies around that peak of the human eye’s sensitivity curve (589 nm). The authors have therefore set as a first criterion that the new candidates must be efficient emitters in the range 380 nm – 580 nm. The reason for selecting this particular range is the need to eliminate any Stoke losses that would be associated with photons the human eye cannot detect and would need conversion with the use of a phosphor. On the short wavelength side the eye sensitivity and therefore the visible range starts at around 380 nm. On the long wavelength side the authors felt that there was no need to include wavelength beyond 580 nm as sodium is already a strong emitter just above that limit, the sensitivity of the eye drops and in any case the photons on that part of the spectrum cannot be converted with the use of phosphor. On the other hand strong emissions in the blue region could be partly converted in order to cover larger regions of the visible.
2.3. The atomic emissions
The entire periodic table was scanned looking for elements with strong lines in the predefined spectral range. A number of elements were excluded from the list not just due to unsuitable emissions but also due to their hazardous nature and disallowed use such as the entire actinide series apart from thorium. Other elements that have been excluded due to European Union directives on hazardous substances (Reduction Of Hazardous Substances, RoHS) are mercury, lead, cadmium and chromium.
2.4. The molecular emissions
After having short listed the elements of the periodic table according to our selection rule that demands for transitions from the lowest lying atomic levels that terminate to the ground state, to result in emissions with wavelengths in our desired range of about 380 – 580 nm, the next step was to look for diatomic species, that contain at least one of the atoms from our short list and satisfy the same selection rule.
2.5. The vapour pressure
As it has already been mentioned, in high pressure discharge lamps where the gas temperature reaches thousands of degrees Celsius, almost any compound would be vaporized to a significant extend that would allow it to play a role in the lighting performance of the lamp. In this study we concentrate on low pressure situations where the gas temperature does not exceed a few hundred degrees Celsius and that the cold spot of the tube could be significantly lower than that. We therefore set another selection rule that demands for the candidate active medium, or its parent compound if different, to be present in the vapor phase at a pressure of at least 0.1 Pascal (0.75 milliTorr) at 300 degrees Celsius or less. These values are only indicative and have been chosen bearing in mind the conditions of the two most known low pressure discharge lamps, those of mercury and sodium vapors.
The list has been further narrowed down to a few species whose parent compounds satisfy the predefined conditions of vapour pressure under a certain temperature according to published values.
In case the diatomic species that reach the final stage of the selection procedure do not exist in a stable form then a parent compound will be selected part of which is the active medium of choice. The selection rule we set at this point is that the molecules chosen must be in the lowest possible oxidation state that still vaporizes to the desired degree in order to avoid stoichiometrically driven condensation. What is meant by this statement can be explained with an example. Consider a metal M that vaporizes to the desired degree when in form of MX4 where X is a halogen or an oxygen atom (or a combination of both as in some oxohalide compounds) but condenses when MX2 forms, then in the plasma phase of a lamp that starts with MX4, all metal will in time condense as it forms MX2 and X2 in the gas phase (such as molecular oxygen or halide). On the other hand if the products of the plasma reactions do not form stable species with very low vapor pressures under those conditions, this problem can be avoided.
Considering the final table of diatomic species that could emit in the spectral range of interest (due to both diatomic and elemental electronic properties), the parent compounds of the diatomics that satisfy the vapour pressure criteria and the final argument that deals with stoichiometrically driven condensation, we come up with a very short list of candidate compounds.3. The experimental testing
The spectrometer employed for the recording of some preliminary spectral information was an Ocean Optics USB2000 with a bandwidth of 1.3 mm, a grating of 600 grooves / mm and a slit of 10 mm. The spectra recorded were averaged for about 100 milliseconds. The detector inside the spectrometer is a CCD array of 2048 elements in one row. The temperature of the outside surface was also measured at two different points (above the electrode and the middle of the tube) using a K type thermo couple. Finally the luminance (L) was measured with a Topcon BM-7 luminance meter placed 50 cm away from the lamp and set at 0.2 degrees field of view (measured area: ~Æ1.7 mm). The meter consists of the focusing lens, aperture for field of view (FOV) setting and three silicon photodiodes (PDs) with filters whose spectral transmittance are adjusted to the human eye's spectral tri-stimulus values (x(l), y(l), z(l)). The luminance and chromaticity coordinates (x, y) are displayed on the luminance meter, which should be calculated in the instruments as (corresponding silicon PD output) / [(FOV) * (measurement area)] * (calibration coefficient).
4. Discussion
The spectral information reveal what indeed our design of the selection rules aimed at, which is for the metal atoms to emit their atomic lines in the blue part of the visible spectrum and for some molecular bands to also appear in the same region covering as much of the visible spectrum as possible.
Acknowledgements
The authors would like to thank the Greek Ministry of Development and the General Secretariat of Research and Technology for funding the project as part of the ENTER initiative as well as professor Georges Zissis of the Centre for Plasma Applications and Technologies of the University of Toulouse for co-funding the project.
This work is co-funded by:
Curriculum Vitae |
Dr Spiros Kitsinelis Doctor of physical chemistry and spectroscopy |
 |
| 1995 - 1999 | Master of Chemistry specializing in visible and ultraviolet spectroscopy as well as light sources of low pressure discharges (University of Sheffield, United Kingdom). Master’s thesis: “Pulsed Operation Of Low Pressure Gas Discharges”. Second Upper Class degree Analytical chemist at central laboratories of oil company ELAIS (01/07/1997 – 15/09/1997) |
| 1999 - 2002 | Doctorate with specialization on plasma light sources (University of Sheffield, United Kingdom) 2 patent applications. Doctorate thesis: “Optical Emission Spectroscopy of Pulse Operated Low Pressure Gas Discharges” Laboratory supervisor, Chemistry department of University of Sheffield, United Kingdom (01/10/1999 – 05/12/2002) |
| 2002 – 2003 | Research and development, Sheffield University Enterprises Limited / Luminaries (17/02/2003 – 15/08/2003) |
| 2003 - 2005 | Postdoctoral research and development of light sources of high efficacy and environmentally – friendly (Ehime University, Japan) 1 patent application |
| 2005 - 2006 | Project leader, Research and Development of novel light sources and dynamic lighting (Philips Lighting, The Netherlands) 4 patent applications |
| 2006 – | Research and development of novel light sources, Physics department, Technical University of Athens, Greece Consultant of Philips Lighting Academy Greece (03/2006 – 06/2006) |
Research areas
Plasma Physics, Electrical discharges and light sources
Publications
Other professional activities
05/12/2003: Invited lecture on “Optical Emission Spectroscopy of Low Pressure Gas Discharges” by the Institute of the Electrical Engineers of Japan (IEEJ)
External evaluator for the COST Action 529 “Lighting for the 21st Century”
Awards Received
Links |
|
||
|
||
|